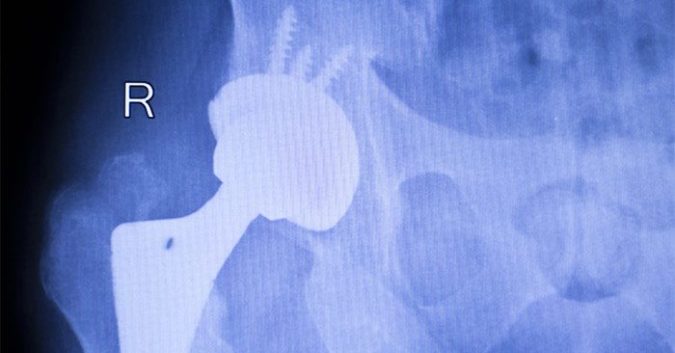
Following Stryker’s voluntary recall of its LFIT™ Anatomic CoCr V40 Femoral Head last August, outrage against the company has been enormous. Patients using this artificial hip replacement product have experienced life-altering difficulties such as pain, loss of mobility, dislocation, and a host of other dangerous side effects.
LFIT was the third product manufactured by Stryker, a leader in the hip implant system industry, to be recalled in 5 years. In that time, the company has received north of 100 complaints: a number that continues to rise steadily.
Hip implant surgery, of course, is a delicate procedure. Surgeons are faced with a puzzle of various components to find the best fit for each individual and, as in any complicated operation, things can go wrong. But no patient should ever have to endanger themselves with side effects this extensive.
Stryker Risks: Here’s What You Might Have Missed
While on the market, LFIT Anatomic Femoral Heads promised to provide “substantial benefits to the surgeon, patient, and hospital.” Their aim was to address common factors associated with hip replacement surgery failure – dislocation, strength, and wear – and the resulting costs. With LFIT’s state-of-the-art design, patients could enjoy minimized risks of dislocation, increased range of motion (ROM), and enhanced hip stability.
The reality, however, was quite different. The femoral head, which is essentially the ball component of the “ball and socket” connection in a hip implant, is supposed to be connected securely to the femoral neck by a taper lock to create a makeshift joint. But instead, numerous events have occurred where the taper lock has botched. In some cases, the connection can weaken due to corrosion, and in others, it can break completely.
Patients have suffered such awful injuries from this defect – sometimes even resulting in broken bones and disabilities – that some have required immediate revision surgery. The defective taper lock has even put patients at risk of metallosis, a type of metal poisoning caused by the release of metal debris into the bloodstream.
This, despite Stryker knowing for years that the femoral heads were dangerous and deciding not to direct doctors to inform their patients of the defects.
The recall only applies to femoral heads produced before 2011 – an estimated 100,000 components – most of which are already implanted in patients. This means that, for many, it’s too late to reverse the damage. Now it’s just a question of what can be done to clean up Stryker’s mess.
The Latest: Lawsuits on the Rise
Stryker is currently under investigation by the Food and Drug Administration (FDA). The company also issued an updated recall in October 2016 after identifying additional customers and lot numbers. But amidst the potentially lengthy investigation process, patients have taken the matter into their own hands by seeking legal action.
Product liability cases against Stryker began as early as 2014, when patients first started discovering problems. As of January 2017, there are at least 6 lawsuits pending in federal courts. But this litigation has the capacity to be much larger, experts say, perhaps warranting a centralized approach in a single U.S. District Court to improve judicial efficiency.
So the wheels of justice are turning slowly, but surely. In the meantime, 1 takeaway from these adverse events with Stryker is that you can never be too careful. Be sure to talk your options through with your doctor and learn of all potential risks before you make decisions. But the consequences of surgery can’t always be foreseen. In these unfair cases, seeking justice is the best patients and their families can hope to do.
If victims are vigilant in pursuing justice, then Stryker may be unable to strike again with defective products, which is a real possibility. This isn’t the first time the company has put patient safety on the line. But with meaningful action to prevent any further catastrophes, can it be the last?