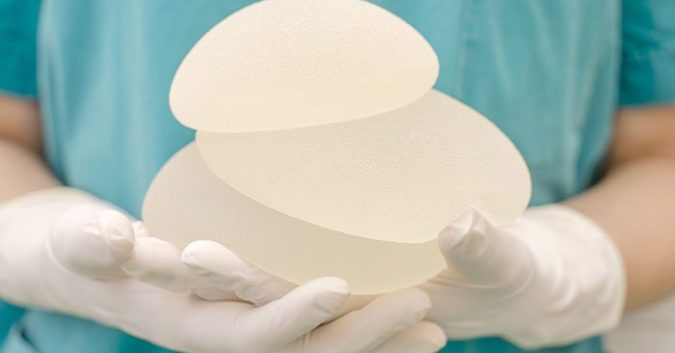
In the past several years, 9 women who underwent breast augmentation procedures died from a rare form of cancer. The cancer, is called anaplastic large cell lymphoma – or ALCL. At first, the U.S. Food and Drug Administration (FDA) didn’t have enough data on at first to be able draw a connection between it and breast implants. didn’t fully understand. Now, this disease has finally made it onto the federal agency’s checklist of potential risks.
This week, the FDA made an official warning that breast implants can cause ALCL, a rare form of non-Hodgkin’s lymphoma. Since the agency first discovered a possible association in 2011, they received 359 medical device reports (MDRs) detailing injuries and deaths caused by the cancer.
Why Are Breast Implants Making Women Sick?
The FDA found that breast implant-associated ALCL occurs more often from implants with textured surfaces rather than smooth (203out of the 359 cases). Reasons for this remain unknown. The breast implant filler material could also be a factor; 186 women’s implants were filled with silicone while 126 had implants filled with saline.
World Health Organization (WHO) researchers also identified more cases of the disease in patients undergoing implant revision operations for late onset problems such as seroma, (collections of excess fluid beneath the skin). In these cases, BIA-ALCL can take up to 10 years to develop.
These cases were documented when women complained of symptoms such as lumps, pain, swelling, and fluid build-up. But of the total 1.7 million breast implant surgeries performed in the U.S. between 2011 and today, the FDA has no precise idea of how many other cases may exist.
It’s important to note that breast implant-associated ALCL is an extremely rare condition. But in order to gather more information for medical research, raise awareness among women, and improve the lives of those already affected, it’s vital to report every case. Not only that, but manufacturers of the implants – who have put so many women’s health in danger – need to be held accountable.
Manufacturers Sued Over Implant Leaks
Many will be unsurprised to find that Johnson & Johnson, the infamous medical device giant, is 1 of the players impacted by litigation.
Last year, a Seattle woman filed a case against Mentor Worldwide, a supplier of surgical aesthetics products acquired by J&J. The product in question was its Mentor MemoryGel™ Silicone Breast Implants, which allegedly had known, dangerous defects the company failed to warn about. According to the lawsuit, certain chemicals that were used during in the implants’ manufacturing leaked into the victim’s body and caused skin rashes, extreme fatigue, and metal poisoning – risks J&J was aware of before her procedure.
Another case surfaced last month in California. Again, allegedly failing to follow FDA requirements, J&J sold its faulty silicone implants to a woman who suffered pain, nausea, skin rashes, and fatigue.
Following the lift of the FDA’s silicone-based implant ban in 2006, Mentor monopolizes sales of silicone implants – part of a $635 Million U.S. breast implant market – along with Allergan Plc and Sientra Inc.
Although the FDA has found stronger evidence that implant texture causes BIA-ALCL, they cannot yet discount the possibility of a causal relationship with silicone. Mentor implants have so far been used in surgeries of 2 million women worldwide.
What Happens Now?
The FDA continues its investigation into ALCL in women with breast implants and treatment options to advise doctors on necessary care. Women who already have implants, the agency suggests, do not need to change their routine care pattern but should pay attention to any changes. To prospective patients, the FDA recommends exploring all information available about breast implant types and to give textured-surface implants serious consideration.
Women with the disease can be treated by removal of the implant and its surrounding tissue. Those with more severe cases, however, may need chemotherapy and radiation.
Treatment may take away the grueling symptoms women have had to experience from defective breast implants – but it doesn’t right the wrongs of their graceless manufacturers. Litigation, at least, can go a long way in shutting their crooked operations down.